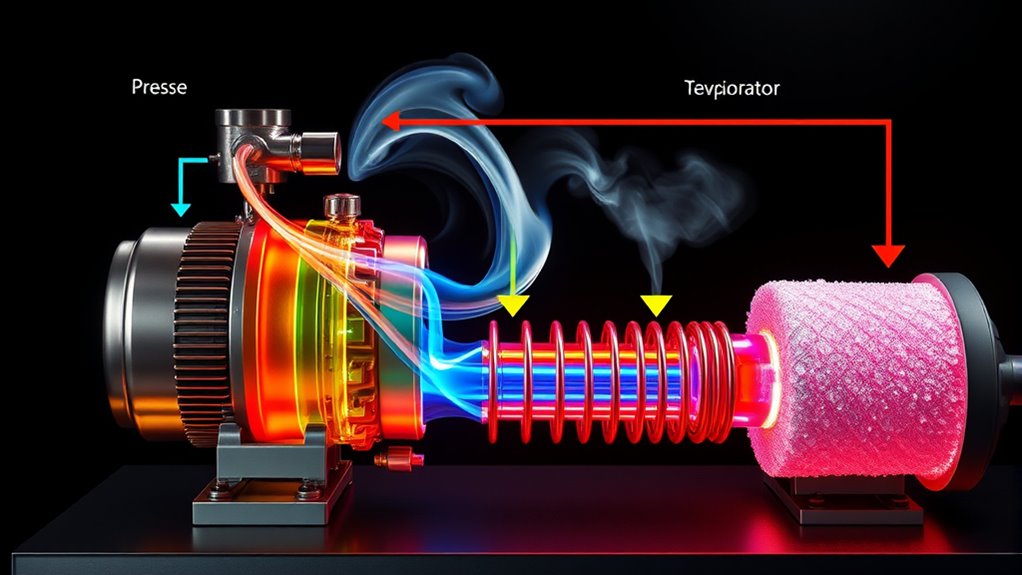
The vapor compression cycle is based on core thermodynamic principles like energy transfer, phase changes, and entropy. You compress low-pressure vapor to high pressure, increasing its temperature, then transfer heat in the condenser. Throttling causes a pressure drop, increasing entropy and preparing the refrigerant for absorption in the evaporator. Understanding these processes helps optimize efficiency and performance—continue to explore how each stage interacts for an all-encompassing grasp of this cycle.
Key Takeaways
- The cycle operates based on the first law of thermodynamics, involving energy transfer through work and heat interactions.
- Compression raises refrigerant pressure and temperature, following the ideal gas law and thermodynamic principles.
- Heat rejection in the condenser occurs when high-pressure vapor condenses into liquid, releasing stored internal energy.
- The expansion valve causes an adiabatic pressure drop, increasing entropy and enabling efficient heat absorption in the evaporator.
- Entropy analysis identifies irreversibilities, guiding optimization to improve cycle efficiency and minimize energy losses.

Have you ever wondered how energy moves and transforms within physical systems? When it comes to the vapor compression cycle, understanding these processes is essential. At its core, this cycle relies on the principles of thermodynamics, where energy transfer and conversion occur through various stages. One key aspect to consider is entropy analysis, which helps you understand the direction and efficiency of these energy changes. Entropy, in simple terms, measures the disorder or randomness within a system. As the refrigerant moves through the cycle, entropy increases in certain parts, indicating irreversibility and energy dispersal. Recognizing where entropy rises allows you to pinpoint inefficiencies and optimize system performance.
The cycle begins with the compressor, where the refrigerant is compressed from a low-pressure, low-temperature vapor into a high-pressure, high-temperature vapor. This compression involves a phase change in the refrigerant, where it transitions from a vapor to a superheated state. As you analyze the phase change occurring here, you’ll notice that the refrigerant absorbs work from the compressor, increasing its internal energy. The high-pressure vapor then flows into the condenser, where it releases heat to the surroundings. During this process, it undergoes another phase change, condensing from a vapor into a liquid. This phase change is essential because it allows the refrigerant to transfer heat efficiently, cooling the environment.
Next, the liquid refrigerant passes through an expansion valve, where its pressure drops suddenly. This pressure drop causes the refrigerant to partially vaporize and cool rapidly—a process known as throttling. Here, you observe a significant entropy increase because the refrigerant’s molecules become more disordered as it transitions from a high-pressure liquid to a low-pressure, mixed-phase mixture. This phase change is fundamental for the cycle to continue, as the cooled refrigerant can absorb heat again when it evaporates in the evaporator. In the evaporator, the refrigerant absorbs heat from the space you’re cooling, causing it to vaporize once more. This completes the cycle, returning the refrigerant to the compressor to repeat the process.
Throughout these stages, phase changes are central to energy transfer and system efficiency. By analyzing entropy at each point, you can identify where energy losses occur and how to minimize them. These insights help you design more effective and energy-efficient vapor compression systems, ensuring better cooling performance while reducing waste. Understanding the interplay of phase change and entropy analysis empowers you to optimize thermodynamic cycles, making your systems more reliable and sustainable.
Frequently Asked Questions
How Does Refrigerant Choice Affect Cycle Efficiency?
Your choice of refrigerant directly impacts cycle efficiency because different refrigerants have varying thermodynamic properties. Using a refrigerant with a suitable boiling point and high latent heat improves heat transfer, reducing energy consumption. Additionally, selecting environmentally friendly refrigerants with low Global Warming Potential (GWP) can enhance efficiency while minimizing environmental impact. Proper refrigerant selection guarantees peak performance, lowers operating costs, and maximizes the overall efficiency of the vapor compression cycle.
What Are Common Cycle Design Mistakes to Avoid?
Avoid common cycle design mistakes by focusing on leak detection and compressor sizing. You should regularly check for leaks to prevent refrigerant loss and guarantee system efficiency. Proper compressor sizing is essential; an undersized compressor struggles to meet cooling demands, while an oversized one wastes energy. By carefully balancing these aspects, you optimize performance, reduce operational costs, and extend equipment lifespan. Don’t overlook these details to achieve a reliable, efficient vapor compression cycle.
How Do Real-World Inefficiencies Impact Theoretical Models?
Real-world inefficiencies, like imperfect heat transfer and system losses, considerably impact theoretical models. They cause the actual cycle to perform below ideal predictions, reducing cooling efficiency and increasing energy consumption. You’ll notice that heat transfer isn’t always the best, and components might introduce additional losses, which means your system won’t operate at its highest potential. Accounting for these inefficiencies helps you design more realistic, effective systems that better match real-world conditions.
Can Cycle Performance Be Optimized for Variable Loads?
Think of your vapor compression cycle as a musical instrument that needs tuning for different performances. You can optimize cycle performance for variable loads by implementing variable load adaptation and performance modulation techniques. These adjustments help your system respond efficiently to changing demands, maintaining cooling capacity and energy efficiency. By fine-tuning the cycle, you ensure it plays harmoniously across all load conditions, maximizing performance and reducing energy consumption.
How Does Ambient Temperature Influence System Capacity?
Higher ambient temperatures decrease your system’s capacity because the condenser needs to dissipate more heat, making it harder for the refrigerant to reject thermal energy efficiently. Conversely, lower ambient temperatures improve capacity, allowing your system to cool more effectively. You should expect reduced performance on hot days and guarantee your system is properly sized and maintained to handle temperature fluctuations for maximum operation.
Conclusion
By understanding the thermodynamic principles behind the vapor compression cycle, you reveal the hidden symphony of heat and work dancing together. It’s like mastering a delicate balance beam—each stage flowing seamlessly into the next, orchestrating cooling with precision. When you grasp these concepts, you’re not just observing a cycle; you’re wielding the very heartbeat of refrigeration technology, turning complex science into a powerful tool that keeps our world comfortably in harmony amid the chaos of thermal energy.